-
所属(所属キャンパス)
-
理工学部 生命情報学科 生命情報学科 ( 矢上 )
-
職名
-
教授
加納 英明 ( カノウ ヒデアキ )
Kano, Hideaki
|
|
マルチモーダル非線形光学顕微鏡

マウス脳切片のCARSイメージ
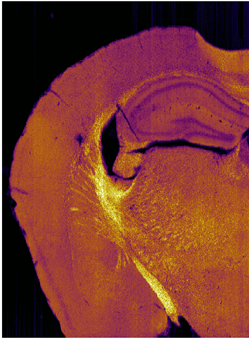
生命現象は、その機能発現に様々な生体分子が協働する複雑な物理化学現象です。生細胞内では、このようなダイナミックな現象が“ごく当たり前”に起こっており、その究極的な理解には、機能する分子をそのまま可視化することが必須です。ラマン分光法は、生細胞内にある分子分布や分子構造、動態を、非染色・非標識・非破壊・低侵襲でその場観察可能な、非常に強力な手法の一つです。私たちは、微弱なラマン散乱光を増幅する非線形ラマン散乱を用い、分子集合体から生細胞・生体組織まで、様々な系を「化学の眼」で可視化し、未知の生命現象の発見とその本質の解明に挑んでいます。
Coherent Anti-Stokes Raman Scattering (CARS) Microscopy and its Applications in Life Sciences
Miyazaki S., Murakami Y., Honjoh S., Hayashi Y., Kano H., Raman Spectroscopy in Human Health and Biomedicine, 2023年01月
Imai R., Kano H., Hattori T.
Analytical Chemistry 97 ( 15 ) 8322 - 8328 2025年04月
ISSN 00032700
ms-Time Resolution Raman Spectroscopy Using sCMOS Cameras
Carpenter A.P., Goulden J., Varagnat A., Klement N., Browne W.R., Kano H.
Proceedings of SPIE - The International Society for Optical Engineering 13348 2025年
ISSN 0277786X
Murakami Y., Obuchi M., Kamizawa H., Miyazaki S., Kishimura A., Oketani R., Hiramatsu K., Leproux P., Hayashi Y., Shiraki K., Kano H.
Analytical Sciences 2025年
ISSN 09106340
Klement W. . ., Leproux P., Browne W., Kano H.
Journal of Raman Spectroscopy 2025年
ISSN 03770486
Ishibashi S., Inoko A., Oka Y., Leproux P., Kano H.
Scientific Reports 14 ( 1 ) 2024年12月
Tribute to Hiro-o Hamaguchi: Expanding the Boundaries of Raman Spectroscopy
Kano H., Bonn M., Zanni M., Tahara T.
Journal of Physical Chemistry B (Journal of Physical Chemistry B) 128 ( 4 ) 883 - 885 2024年02月
ISSN 15206106
心アミロイドーシスの早期診断・早期治療に向けた分光特性と細胞病態の相関理解
加納 英明, 基盤研究(B), 補助金, 研究代表者
第二高調波による細胞内らせん構造のラベルフリー新機能探索
加納 英明, 挑戦的研究(萌芽), 補助金, 研究代表者
生命システム情報特別講義A
2025年度
生命情報特別講義第1
2025年度
生命情報輪講
2025年度
生命現象の物理学
2025年度
生命系の物理化学第2
2025年度